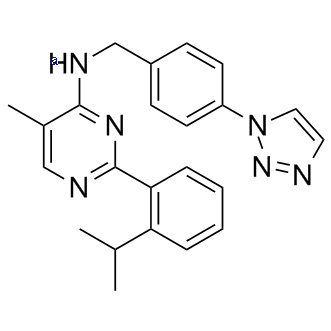
Chemical Information
| M.Wt |
384.48 |
Storage |
Please store the product under the recommended conditions in the Certificate of Analysis. |
| Formula |
C23H24N6 |
| CAS No |
1572414-83-5 |
| Solubility |
DMSO
|
Biological Activity of ML-323
ML323 is a reversible, potent USP1-UAF1 inhibitor with IC50 of 76 nM in a Ub-Rho assay and 174 nM and 820 nM in orthogonal gel-based assays using K63-linked diubiquitin (di-Ub) and monoubiquitinated PCNA (Ub-PCNA) as substrates, respectively.
IC50 Value: 76 nM(in Ub-Rho assay) [1]
Target: USP1-UAF1
in vitro: ML323 probably exerts its inhibitory effect through an allosteric mechanism. The measured inhibition constants of ML323 for the free enzyme (Ki) and the enzyme-substrate complex (K′i) were 68 nM and 183 nM, respectively, which is in accordance with the IC50 determination. ML323 showed little to no inhibition against the USPs tested, including USP2, USP5, USP7, USP8, USP10, USP11, USP14 and USP21. Notably, ML323 did not inhibit the USP46-UAF1 complex that contains the same UAF1 subunit as the USP1-UAF1 complex and demonstrated little inhibition against USP1 alone (IC50 > 200 μM) in a Ub-Rho assay in which USP1-UAF1 and USP1 demonstrated similar Km values. Furthermore, ML323 displayed little to no inhibition against DUBs in the ubiquitin C-terminal hydrolase (UCH), ovarian tumor protease (OTU) and Machado-Joseph domain (MJD) families and did not inhibit a deSUMOylase, SENP1, and a deneddylase, NEDP1. ML323 inhibits USP1-UAF1 through a mixed inhibition mechanism. inhibition of cellular USP1-UAF1 activity by ML323 compromises Polη-mediated TLS of UV-induced DNA damage. ML323 led to a similar decrease in LD50 in both PD20 and PD20 + D2 cells (to 2.8 and 3.4 J m?2, respectively) [1].
in vivo:
[1]. Liang, Q., et al., A selective USP1-UAF1 inhibitor links deubiquitination to DNA damage responses. Nat Chem Biol, 2014.
Abstract
Protein ubiquitination and deubiquitination are central to the control of a large number of cellular pathways and signaling networks in eukaryotes. Although the essential roles of ubiquitination have been established in the eukaryotic DNA damage response, the deubiquitination process remains poorly defined. Chemical probes that perturb the activity of deubiquitinases (DUBs) are needed to characterize the cellular function of deubiquitination. Here we report ML323 (2), a highly potent inhibitor of the USP1-UAF1 deubiquitinase complex with excellent selectivity against human DUBs, deSUMOylase, deneddylase and unrelated proteases. Using ML323, we interrogated deubiquitination in the cellular response to UV- and cisplatin-induced DNA damage and revealed new insights into the requirement of deubiquitination in the DNA translesion synthesis and Fanconi anemia pathways. Moreover, ML323 potentiates cisplatin cytotoxicity in non-small cell lung cancer and osteosarcoma cells. Our findings point to USP1-UAF1 as a key regulator of the DNA damage response and a target for overcoming resistance to the platinum-based anticancer drugs.




 Apoptosis
Apoptosis
 Aurora Kinase
Aurora Kinase